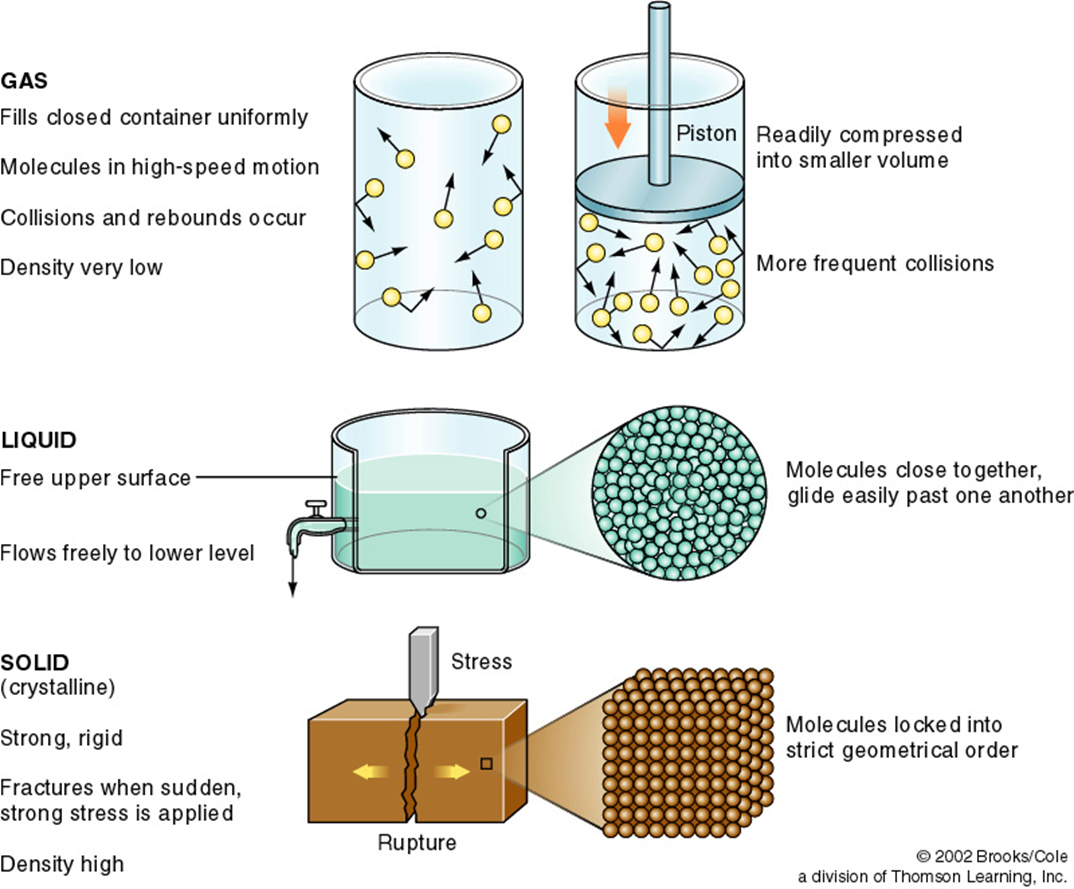
Phases of Substances
The Water Molecule
All elements want two things: to be electrically neutral and to have 8 electrons in their outermost (valence) shell. Most elements don't have these items, so they combine with other elements to form molecules. This is the case for the water molecule. Hydrogen (H+) only has one electron and a charge of +1. Oxygen (O2) has eight electrons, but only 6 are in the valence shell, which creates a -2 charge. Two hydrogen molecules combine with one oxygen molecule and they all share their electrons, so everyone has 8 electrons in the valence shell, and the molecule is electrically neutral (+1++1+-2 = 0).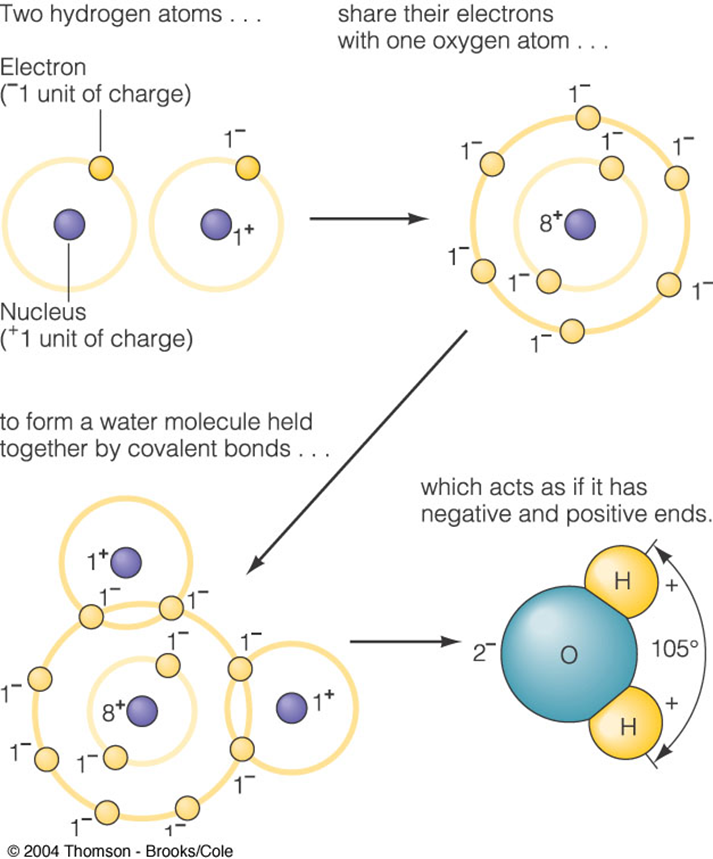
However, the hydrogen atoms are preferentially located on one side of the oxygen atom, making the molecule look like Mickey Mouse Ears. This creates a net positive charge on the side where the hydrogen atoms are located, and a net negative charge on the opposite side. These charges make the molecule act somewhat like a magnet, allowing the positive end to attach itself to negatively charged ions (anions) and the negative end to attach to positively charged ions (cations). Thus, the water molecule is said to be a polar molecule, and this gives it many unique properties.
Properties of Water
Cohesion - the ability of water molecules to stick to each other, creating surface tension. This cohesive force draws the water molecules together, creating a "film" which makes it difficult for objects to penetrate. Water has a very high surface tension.
Adhesion – the tendency of water molecules to stick to other substances. This allows the molecule to attach itself to other ions, dissolving them into the solution. .Thus, water can act as a solvent
People tend to use the terms heat and temperature interchangeably, but they actually have two different meanings. Heat is energy produced by the random vibrations of atoms or molecules. Temperature is an object’s response to input or removal of heat. So, as molecules vibrate more, they generate more heat and therefore the temperature of the molecule is raised.
Heat capacity is the measure of the heat required to raise the temperature of 1 gram of a substance by 1° C. Metals and rocks have a very low heat capacity, which means that they heat up and cool down quickly. Water has a very high heat capacity. This means that it is slow to heat up and slow to cool down. This is the first method our planet uses to moderate it's temperature. Because it takes so long for water to heat up / cool down, water tends to stay in it's liquid phase.
All substances can move from one phase to another (ie, gas -> liquid -> solid), but it takes energy to do so. The amount of energy required to break the bonds is termed the latent heat of vaporization. Water has the highest latent heat of vaporization of any known substance, which is another reason why it tends to stay as a liquid. It takes 80 calories to go from a solid (ice) to freezing water, then 100 calories to go from freezing to boiling water (liquid) then 540 calories to go from boiling water to water vapor. What to go the other direction? Then you need to remove that many calories to go from one phase to another. This is the second method Earth uses to moderate it's temperature: it takes a lot of energy for water to go back and forth from a liquid to a gas.
Therefore, it resists changing temperature when heat is added or removed.
