Properties of Seawater: Salinity
Salinity is the total amount of solid, inorganic material dissolved in water. Salinity levels are typically expressed as 0/00 or parts per thousand (ppt). If you were to take the surface salinity levels for all the world's oceans, the average level would be 35 0/00.
The heat capacity of water decreases with increasing salinity. Thus, as the salinity increases, the boiling & freezing points decrease and evaporation slows. Fresh water freezes at 32° F (0° C). The increase in salinity will therefore allow the water to freeze at a lower temperature (say, 25° F). The same is true for the boiling point (212° F) - the addition of salts will lower the boiling point. This is the reason why you are directed to add salt to water when making pasta. The salt lowers the boiling point of the water, allowing the water to come to a boil more quickly. It's not to add flavor. If you're adding enough salt to taste it in the water you're adding too much!
Osmotic pressure increases as salinity increases. What is osmotic pressure? Wikipedia defines it as "the minimum pressure which needs to be applied to a solution to prevent the inward flow of water across a semipermeable membrane". An example is what happens to your skin when you take too long in a bubble bath. Your body is more saline that the water you're sitting in, so your body tries to balance the pressures by releasing salts from your body into the surrounding water. What this means in the ocean is that organisms are adapted to live at certain depths, temperatures, and salinity levels. Moving them out of those conditions can cause them to die. Thus you can't put freshwater fish in a salt water tank, and visa versa!
If you have 1000 grams of saltwater, 965 of those grams are plain water. The remaining 35 grams are the "salts".

A Salt = cation + anion. Cations have a positive charge (Na+) while anions have a negative charge (Cl-).
"Salts" in seawater include the following: Na+, Cl-, SO42-, Mg2+, HCO3-, Ca2+, and K+
Note all those charges on those elements. Those charges allow for the water molecule to attach itself to them; the positive end of the water molecule will attach itself to the negative chloride ion (Cl-) and the negative end to the positive sodium ion (Na+). In this way ions are dissolved into the water, making it a saline solution. As long as there are more water molecules than ions, the ions will be dissolved into the solution.
Remember . . .
 |
 |
Is the ocean becoming progressively saltier with age?
No! The ocean is in chemical equilibrium. The proportion and amounts of dissolved solids remain constant. This is known as the “steady state ocean.” Basically, what goes in = what goes out.
So . . .what does go in or out? Salts going in must equal salts going out!
| Salts come from: | Salts removed by: |
| Anions - Volcanic gasses Cations - Minerals in rocks | Sea spray Evaporates Biologic processes Magma at Mid-Ocean Ridges |
Desalination
Making fresh water from salt water (Expensive)
Methods:
- Change of state
- Electrodialysis
- Ion exchange
Important Factors that either increase or decrease salinity
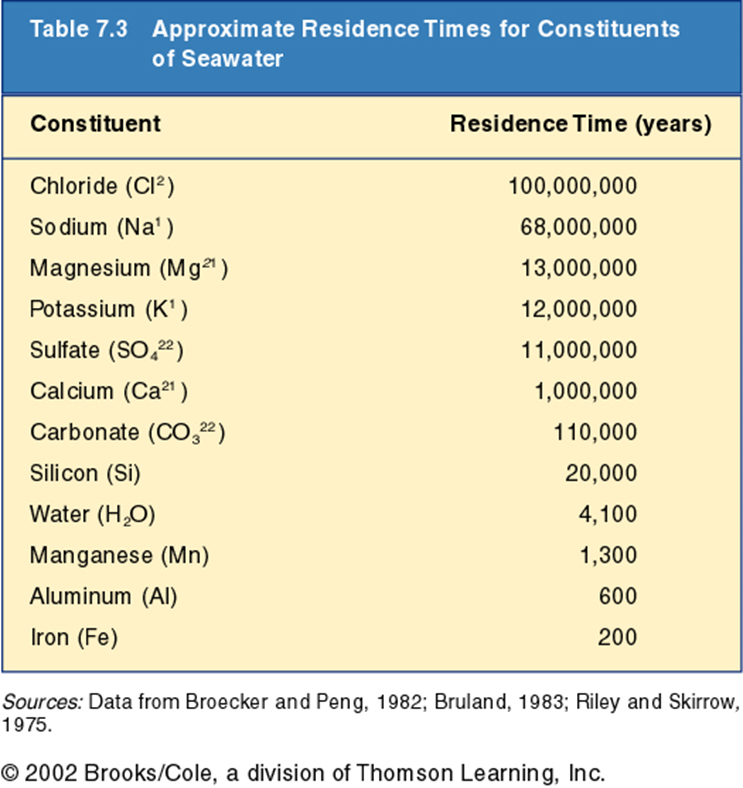
Residence time
Residence time is, essentially, how long an element stays in the ocean. Some elements have loooong residence times (like Na+ & Cl-) and others very short times. The elements that have the longest residence times are those that comprise the "salts" in the ocean.
How is the salinity level calculated?
Salinity is calculated by taking the chlorinity levels (in ppt) and multiplying it by 1.80655. This can be bothersome as it's not always easy to determine the chlorinity of the water. Instead, most scientists use a devise called a salinometer, which will automatically determine the salinity levels for you.
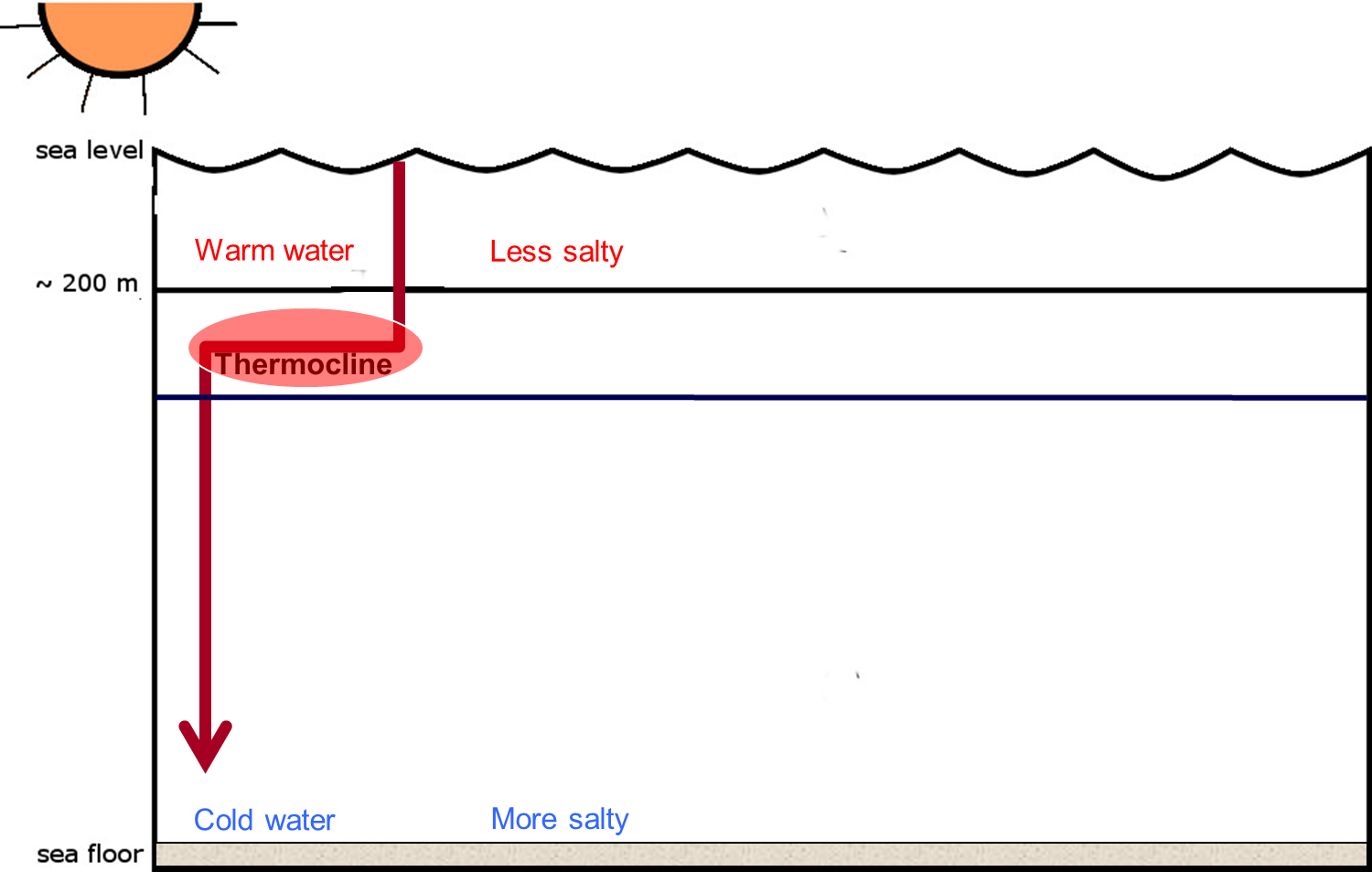
Does the salinity levels change with depth in the ocean?
Yes! Warm water is less salty that water that is cold. Thus, there is layering in the ocean with respect to salinity levels. Surface waters are warm and less salty, while deeper waters are colder and more salty. And, as with temperature, there is a shift from less to more saline called the halocline.
Check out the picture below!