Properties of Seawater: Dissolved Gasses
Physical Factors
Water Temperature
- Cold water holds more gas in solution than warm
- (this is why we Americans like our beer and soda COLD!)
| Solubility of Gases in Seawater as a Function of Temperature | |||
| Solubility (1 ml/l at 1 atm) | |||
| Temperature (°C) | N2 | O2 | CO2 |
| 0 | 14.47 | 8.14 | 8,700 |
| 10 | 11.59 | 6.42 | 8,030 |
| 20 | 9.65 | 5.26 | 7,350 |
| 30 | 8.26 | 4.41 | 6,660 |
Partial pressure of the gasses in the atmosphere
The higher the pressure of these gasses in the atmosphere, the more gas is held in solution. Deeper waters = more pressure = more gas in solution

Biological Factors
- Photosynthesis - Consumes CO2, produces O2 + water
- Respiration - Consumes O2, produces CO2 + water
- Decomposition (Organic matter decay) - Consumes O2, produces CO2 + water
- Organic matter decays; releases CO2 = biological pump
Where do the gasses come from?
- From the atmosphere
- Breaking waves / surf (aeration)
- Volcanic eruptions
- Phytoplankton
O2 and CO2 Levels in the Sea
- Photosynthesis ALWAYS occurs in the photic zone, because that's where the plants are.
- Respiration mostly occurs in the photic zone, because animals that want to eat the tasty plants must go to where the plants are.
- Decomposition occurs at the sea bed, because that's where the dead organisms collect to decay.
Therefore, O2 levels should be high near the surface, and low near the sea floor, and the opposite for CO2 levels.
The amount of each gas varies due to many different factors. Lots of photosynthesis results in lots of O2. The time of day is one factor. Photosynthesis, and therefore O2 levels, are low at night due to a lack of sunlight. Oxygen levels are lowest just before dawn. They are highest at mid-afternoon as photosynthesis has been occurring all morning long.
The season also affects how much O2 is present as well. Levels are lowest in the winter due to the low angle of the sun and less hours of daylight. Levels increase in the spring and max out in the summer as the days get longer and the sun rises higher in the sky.
Acidity Of Seawater
An acid is a substance that releases a hydrogen ion (H+) in solution. A base is a substance that combines with a hydrogen ion (OH-) in solution. Acidity or alkalinity is measured on the pH scale.
pH is measure of Hydrogen ions in water. The higher the pH, more basic or alkali the solution is. The lower the pH, the more acidic the solution is. The more CO2 there is in the solution, the more acidic it is. pH is expressed as numbers, ranging from from 0 (acid) to 14 (base). The values for sea water are 7.5 – 8.4, with 7.8 as the average.
So how does seawater stay close to neutral when there is too much O2 at the surface, which should make the water too basic, and too much CO2 at the seafloor, which should make the water too acidic. The reason is due to the hydrogen ion and the the carbonate buffering system. When the seawater is too acidic, water (H2O) and CO2 to form carbonic acid (H2CO2). However, hydrogen will separate from the carbonic acid molecule to form the bicarbonate ion HCO3- and H+, and the pH drops. If the water is too basic, the equation will work in reverse, and the carbonate ion CO3 will combine with hydrogen to form the bicarbonate ion HCO3-.
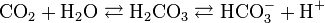
Carbon Dioxide + water ↔ Carbonic Acid ↔ Bicarbonate ion + hydrogen ion
Processes That Affect CO2 Levels
|
Increase CO2 |
Decrease CO2 |
|
|
So, here's our layers of the ocean now. The surface waters are warm, less salty, less dense, low in nutrients, low in CO2 and high in O2 due to photosynthesis by plants. The deeper waters are cold, more salty, more dense, high in nutrients and low in O2 and high in CO2 due to decomposition of organisms on the sea floor.
